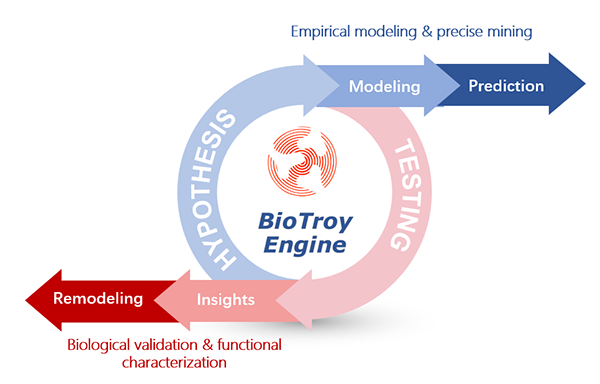
BioTroy Engine
A unique target identification platform has been developed by BioTroy, employing integerated approaches including multi-omics dissection of microenvironment, functional hypothesis-driven modeling and precise mining, immununological functions characterization, and target validation methods.
Using our BioTroy Engine platform, we have developed first-in-class oncoimmunology therapies with the greatest degree of novelty and translational significance.
Antibody discovery & engineering
BioTroy empowers first-in-class IO therapeutics by ensuring high diversity, affinity and developability in the antibody discovery process.
The creative design of antigen preparation, immunization and screening strategies are driven by our insights into the biological functions of the target and relevant physiopathological processes. A highly motivated team led by experts in both oncoimmunology and antibody engineering ensure the unlimited approximity to "best-in-class" molecules.
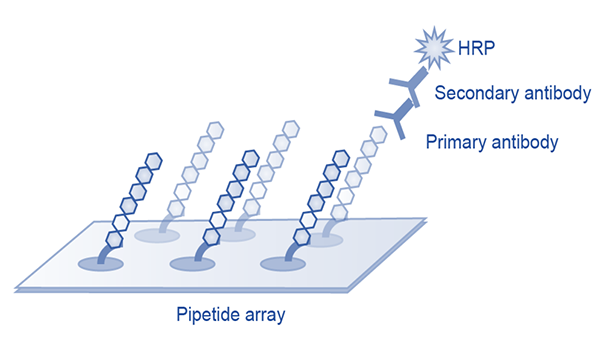
Epitope characterization
Every leading pipeline is covered by multinational patents featuring different aspects of the biologics, including epitopes characterized by x-ray crystallization or peptide arrays.
Chemical Manufacturing and Control (CMC)
BioTroy holds a in-house platform to define approaches for manufacturing processes control, product characteristics, and product testing, in order to ensure that the therapeutic antibody is safe, effective and consistent between batches. BioTroy‘s CMC platform includes stable cell line development, cell culture process development(15L scale) , purification process development, formulation process development and analysis and quality control platform. In 2023 year, we will establish GMP pilot production platform.




